

You can:
| Name | Cannabinoid receptor 1 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | CNR1 |
| Synonym | CB1 Central cannabinoid receptor SKR6R THC receptor CB1R [ Show all ] |
| Disease | Obesity; Diabetes Chemotherapy-induced nausea Diabetes; Obesity Drug abuse Hypertension; Diabetes; Obesity [ Show all ] |
| Length | 472 |
| Amino acid sequence | MKSILDGLADTTFRTITTDLLYVGSNDIQYEDIKGDMASKLGYFPQKFPLTSFRGSPFQEKMTAGDNPQLVPADQVNITEFYNKSLSSFKENEENIQCGENFMDIECFMVLNPSQQLAIAVLSLTLGTFTVLENLLVLCVILHSRSLRCRPSYHFIGSLAVADLLGSVIFVYSFIDFHVFHRKDSRNVFLFKLGGVTASFTASVGSLFLTAIDRYISIHRPLAYKRIVTRPKAVVAFCLMWTIAIVIAVLPLLGWNCEKLQSVCSDIFPHIDETYLMFWIGVTSVLLLFIVYAYMYILWKAHSHAVRMIQRGTQKSIIIHTSEDGKVQVTRPDQARMDIRLAKTLVLILVVLIICWGPLLAIMVYDVFGKMNKLIKTVFAFCSMLCLLNSTVNPIIYALRSKDLRHAFRSMFPSCEGTAQPLDNSMGDSDCLHKHANNAASVHRAAESCIKSTVKIAKVTMSVSTDTSAEAL |
| UniProt | P21554 |
| Protein Data Bank | 5tjv, 5u09, 5xr8, 5xra, 6n4b, 5tgz |
| GPCR-HGmod model | P21554 |
| 3D structure model | This structure is from PDB ID 5tjv. |
| BioLiP | BL0384680, BL0364157, BL0384679, BL0384681, BL0384682, BL0384683, BL0384684, BL0440253, BL0440254,BL0440255, BL0363267, BL0361447, BL0361446 |
| Therapeutic Target Database | T76685 |
| ChEMBL | CHEMBL218 |
| IUPHAR | 56 |
| DrugBank | BE0000061 |
| Name | Anandamide |
|---|---|
| Molecular formula | C22H37NO2 |
| IUPAC name | (5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-tetraenamide |
| Molecular weight | 347.543 |
| Hydrogen bond acceptor | 2 |
| Hydrogen bond donor | 2 |
| XlogP | 5.4 |
| Synonyms | N-Arachidonoyl-2-hydroxyethylamide (5Z,8Z,11Z,14Z)- N-(2-Hydroxyethyl)- 5,8,11,14-eicosatetraenamide SCHEMBL43143 5,8,11,14-Eicosatetraenamide, N-(2-hydroxyethyl)-, (5Z,8Z,11Z,14Z)- [3H]Anandamide [ Show all ] |
| Inchi Key | LGEQQWMQCRIYKG-DOFZRALJSA-N |
| Inchi ID | InChI=1S/C22H37NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-22(25)23-20-21-24/h6-7,9-10,12-13,15-16,24H,2-5,8,11,14,17-21H2,1H3,(H,23,25)/b7-6-,10-9-,13-12-,16-15- |
| PubChem CID | 5281969 |
| ChEMBL | CHEMBL15848 |
| IUPHAR | 2364 |
| BindingDB | 22988 |
| DrugBank | N/A |
Structure | 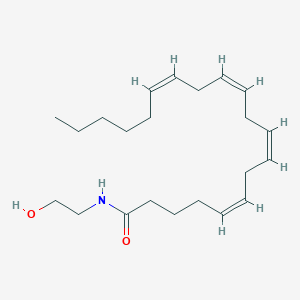 |
| Lipinski's druglikeness | This ligand has a partition coefficient log P greater than 5. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| Ki | 11.0 nM | PMID19361197 | BindingDB,ChEMBL |
| Ki | 39.2 nM | PMID12166938 | BindingDB,ChEMBL |
| Ki | 50.0 nM | PMID16078824 | BindingDB,ChEMBL |
| Ki | 70.0 nM | PMID22044209, PMID16682198, PMID19161308, PMID16570929 | BindingDB,ChEMBL |
| Ki | 72.0 nM | PMID17561406, PMID25065940, PMID16279794, PMID19331413, PMID16213718 | BindingDB,ChEMBL |
| Ki | 89.0 nM | PMID23865723 | BindingDB,ChEMBL |
| Ki | 100.0 - 501.187 nM | PMID7565624, PMID8819477 | IUPHAR |
| Ki | 300.0 nM | PMID14613317 | BindingDB,ChEMBL |
| Ki | 1800.0 nM | PMID14613317 | BindingDB,ChEMBL |
| Log Ki | 1.95 nM | PMID10882356 | ChEMBL |
zhanglab![]() zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417
zhanggroup.org | +65-6601-1241 | Computing 1, 13 Computing Drive, Singapore 117417